Introduction: What is HCOOCH CH₂ H₂O?
Ever wondered what happens when HCOOCH (methyl formate) reacts with CH₂ (methylene or methyl group) and H₂O (water)? While the formula might look confusing at first glance, it actually describes a fascinating chemical reaction with real-world significance. From fuel cells to chemical labs, this simple trio of compounds has a story worth exploring.
Let’s break it all down in a way that’s simple, engaging, and practical.
1. Breaking Down the Formula: HCOOCH + CH₂ + H₂O
HCOOCH Methyl Formate
-
A sweet-smelling liquid.
-
It’s an ester, formed by combining methanol and formic acid.
-
Used in perfumes, solvents, and chemical synthesis.
CH₂ Methylene Group
-
A carbon with two hydrogen atoms.
-
Commonly found in many organic reactions, especially in polymer chemistry.
H₂O Water
-
The universal solvent.
-
Often used to break or form bonds during chemical reactions.
2. The Chemistry: How Do These Compounds React?
The Reaction Process
The most common reaction is hydrolysis where methyl formate reacts with water:
In this reaction:
-
The ester bond in HCOOCH₃ breaks.
-
Water donates a hydrogen (H⁺) and hydroxyl (OH⁻) to create two new molecules.
Role of CH₂
CH₂ might be part of a side reaction or intermediate stage, especially in advanced synthesis. In some contexts, it represents a methyl or bridging group for chain reactions or substitutions.
Read also: HCOOCH CH₂ H₂O: Understanding This Chemical Formula Simply
3. Why Does This Reaction Matter?
Practical Use
-
This reaction is widely used in organic chemistry labs for educational purposes.
-
In industrial settings, it’s a step in producing formic acid and methanol two incredibly useful chemicals.
Real-World Applications
-
Formic acid is used in leather production and rubber manufacturing.
-
Methanol is used as a solvent, fuel, and antifreeze.
4. Industrial Applications of HCOOCH CH₂ H₂O
Fuel Cells
-
Formic acid is a hydrogen carrier in direct formic acid fuel cells (DFAFCs).
-
The reaction that produces it is key to renewable energy systems.
Textile & Leather
-
Formic acid helps with fixing dyes and preserving leather.
Chemical Manufacturing
-
Used in making formaldehyde, acetic acid, and various solvents.
5. Safety Concerns & Handling Guidelines
Methyl Formate (HCOOCH)
-
Flammable.
-
May cause irritation on contact.
Formic Acid
-
Corrosive use: gloves and goggles.
-
Must be stored in a cool, dry place.
General Precautions
-
Always work in a well-ventilated lab.
-
Store chemicals in labeled containers.
-
Avoid direct inhalation or skin contact.
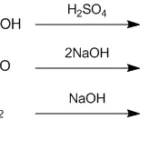
6. Reaction Conditions: Catalysts, Temperature, pH
Catalysts Used
-
Acids like sulfuric acid (H₂SO₄) can speed up hydrolysis.
-
Bases like NaOH also work but follow a different mechanism.
Temperature
-
Moderate heating (around 40°C–70°C) often helps the reaction proceed faster.
pH Range
-
Acidic or neutral pH is preferred for smoother reaction pathways.
7. Environment & Sustainability
Eco-Friendly Potential
-
Since formic acid can be used in hydrogen storage, this reaction plays a role in clean energy.
-
Methanol can be derived from CO₂ using green processes.
Recycling Possibilities
-
Modern research focuses on recycling esters or using enzymes for eco-safe reactions.
8. Research & Future Scope
Catalyst Innovation
-
Enzymes and nanocatalysts are being explored to make reactions greener and faster.
Bio-based Alternatives
-
Producing formic acid and methanol from biomass could replace fossil-based methods.
AI in Reaction Prediction
-
AI is now being used to predict reaction outcomes, especially with complicated ester and water reactions.
FAQs about HCOOCH CH₂ H₂O
Q1: What does HCOOCH mean?
It stands for methyl formate, a common ester made from methanol and formic acid.
Q2: What happens when it reacts with water?
It undergoes hydrolysis and breaks down into formic acid and methanol.
Q3: Is the reaction dangerous?
While the chemicals can be hazardous if mishandled, the reaction itself is safe under controlled lab conditions.
Q4: Where is this reaction used?
In the chemical, textile, energy, and fuel industries.
Q5: Can this be done at home?
No. It requires laboratory safety and should only be done by trained professionals.
Q6: What does CH₂ represent here?
It’s a methylene group that may play a role as a reaction bridge or intermediate.
Conclusion: A Simple Reaction With Big Potential
Though it might seem like a string of random letters HCOOCH CH₂ H₂O this formula represents a reaction full of chemistry, utility, and future possibilities. From powering your devices to helping the environment, its real-world impact is far from small.
Whether you’re a student a chemist or just curious now you know how a small molecule trio can create major results.