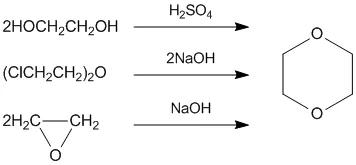
Hey there, chemistry curious friend! Ever stumbled across the formula HCOOCH CH₂ H₂O and wondered what on earth it means? Don’t worry, you’re not alone. Chemical formulas can look like alien language at first glance, but I promise they’re much simpler than they appear once you break them down.
Let’s dive into this fascinating world together. We’ll explore what this formula represents, why it matters, and how it connects to things you might already know. By the end of our chat, you’ll feel much more confident about understanding chemical formulas in general.
What Is HCOOCH CH₂ H₂O?
First things first, let’s decode this mysterious formula step by step. The formula HCOOCH CH₂ H₂O might look complicated, but it’s actually telling us a story about atoms and how they connect together. Think of it like a recipe that shows exactly which ingredients you need and how much of each.
In chemistry, every letter represents a different type of atom. H stands for hydrogen, C means carbon, and O represents oxygen. When we see these letters together in a formula, they’re showing us how these tiny building blocks combine to create something new. It’s like building with molecular Lego blocks.
The subscript numbers (like the ₂ in CH₂) tell us how many of each atom we have. If there’s no number, that means there’s just one of that atom. So CH₂ means one carbon atom connected to two hydrogen atoms. Pretty straightforward once you know the code, right?
Breaking Down the Components
Let’s take a closer look at each part of HCOOCH CH₂ H₂O. This formula actually represents a combination of different molecular pieces working together. Understanding each piece helps us see the bigger picture of what’s happening at the molecular level.Formic acid
The HCOOCH part is particularly interesting because it shows us a specific arrangement of atoms. This grouping is common in organic chemistry, where we study carbon-based compounds. The way these atoms are arranged affects how the whole molecule behaves and what properties it has.
The CH₂ section represents what chemists call a methylene group. This is a super common building block in organic molecules. You’ll find CH₂ groups in everything from plastics to the fats in your body. The H₂O part, of course, is our old friend water – two hydrogen atoms bonded to one oxygen atom.
Why Chemical Formulas Matter
You might be wondering why we even need to understand formulas like HCOOCH CH₂ H₂O. Well, these formulas are like the language of chemistry. They help scientists communicate exactly what they’re talking about without any confusion.
Think about it this way: if I said “that bubbly drink,” you might think of soda, champagne, or sparkling water. But if I give you the exact chemical formula, there’s no guesswork involved. Everyone knows exactly what molecule we’re discussing, no matter what language they speak.
Chemical formulas also help us predict how different substances will behave. Will they mix together? Will they react? Will they be safe to handle? The formula gives us clues about all of these important questions. It’s like having a roadmap for understanding the molecular world around us.
The Role of Hydrogen in Our Formula
Let’s talk about hydrogen for a moment. In HCOOCH CH₂ H₂O, hydrogen appears in multiple places, and each position tells us something different about the molecule’s behavior. Hydrogen is the simplest atom – just one proton and one electron – but it plays crucial roles in chemistry.
Hydrogen atoms love to form bonds with other atoms, especially carbon and oxygen. In our formula, you can see hydrogen bonding with both carbon (in CH₂) and oxygen (in H₂O). These bonds are like tiny springs that hold the molecule together while still allowing some flexibility.
The position of hydrogen atoms also affects how the molecule interacts with other substances. Some hydrogen atoms might be more reactive than others, depending on their neighbors. This is why the exact arrangement shown in formulas like HCOOCH CH₂ H₂O is so important for understanding chemical behavior.
Carbon’s Central Role
Carbon is often called the backbone of organic chemistry, and you can see why when looking at HCOOCH CH₂ H₂O. Carbon atoms have a special ability to form stable bonds with many other atoms, including other carbon atoms. This makes them perfect for building complex molecular structures.
In our formula, carbon appears in different environments. Some carbon atoms are bonded to hydrogen, others to oxygen, and some to other carbon atoms. Each of these arrangements gives the carbon atom slightly different properties and behaviors. It’s like how the same person might act differently at work versus at home.
The versatility of carbon is what makes life possible. From the DNA in your cells to the proteins in your muscles, carbon-based molecules are everywhere. Understanding how carbon behaves in formulas like HCOOCH CH₂ H₂O helps us understand the chemistry of life itself.
Oxygen’s Important Functions
Oxygen brings its own special properties to HCOOCH CH₂ H₂O. This element is highly reactive and loves to form bonds with other atoms. In our formula, oxygen appears in different roles, each contributing something unique to the overall molecule.
Oxygen atoms can form single or double bonds with other atoms. The type of bond affects the molecule’s shape and behavior. Double bonds are stronger but less flexible than single bonds. It’s like the difference between a rigid steel beam and a flexible rope – both are useful, but in different situations.
The presence of oxygen also affects how the molecule interacts with water and other substances. Oxygen-containing groups often make molecules more water-friendly, which is important for biological processes. This is why understanding the oxygen content in formulas like HCOOCH CH₂ H₂O tells us so much about how the substance might behave.
How Molecules Connect and Interact
When we look at HCOOCH CH₂ H₂O, we’re not just seeing individual atoms floating around. These atoms are connected in specific ways that determine the molecule’s overall shape and properties. Think of it like a 3D puzzle where each piece has to fit perfectly with its neighbors.
The bonds between atoms are like invisible springs. They’re strong enough to hold the molecule together, but flexible enough to allow movement and vibration. This flexibility is important because it allows molecules to interact with each other in useful ways.
Sometimes molecules like those described by HCOOCH CH₂ H₂O can form temporary connections with other molecules. These interactions might be weak individually, but together they can create significant effects. It’s like how individual raindrops are tiny, but together they can fill a river.
Real-World Applications
Understanding formulas like HCOOCH CH₂ H₂O isn’t just academic exercise. This knowledge has practical applications in many fields. From developing new medicines to creating better materials, chemical formulas guide real-world innovations.
In the pharmaceutical industry, scientists use molecular formulas to design drugs that interact with specific targets in the body. By understanding how different atomic arrangements affect biological activity, they can create more effective treatments with fewer side effects.
Materials science also relies heavily on understanding chemical formulas. Whether it’s developing stronger plastics, more efficient solar panels, or better batteries, knowing how atoms connect and interact is crucial. The same principles that help us understand HCOOCH CH₂ H₂O apply to countless other important compounds.
Common Misconceptions
Let me clear up some common misunderstandings about chemical formulas like HCOOCH CH₂ H₂O. First, many people think these formulas are impossibly complex, but they’re actually quite logical once you learn the basic rules.
Another misconception is that chemical formulas are just theoretical constructs with no connection to reality. Actually, these formulas represent real physical arrangements of atoms that you could theoretically see if you had a powerful enough microscope.
Some people also think that understanding chemical formulas requires years of study. While becoming an expert does take time, grasping the basics is much more accessible than most people realize. The key is starting with simple concepts and building up gradually.
Learning Tips for Chemical Formulas
If you want to get better at understanding formulas like HCOOCH CH₂ H₂O, here are some friendly tips that have helped many people. Start by memorizing the symbols for common elements like hydrogen, carbon, and oxygen. These appear in most organic compounds.
Practice reading formulas out loud. Instead of seeing HCOOCH CH₂ H₂O as a jumbled mess, try to identify each component. Say “H-C-O-O-C-H, then C-H-two, then H-two-O.” This helps your brain process the information more naturally.
Don’t try to memorize everything at once. Focus on understanding the patterns and logic behind chemical formulas. Once you grasp the underlying principles, individual formulas become much easier to decode and remember.
The Future of Chemical Understanding
As we continue to advance in science and technology, understanding chemical formulas like HCOOCH CH₂ H₂O becomes increasingly important. New discoveries in chemistry are happening all the time, and they all build on these fundamental concepts.
Computer modeling now allows scientists to predict how new molecules will behave before they even synthesize them. This saves enormous amounts of time and resources. But these computer models are based on the same principles we use to understand formulas like HCOOCH CH₂ H₂O.
Artificial intelligence is also revolutionizing chemistry by helping scientists discover new compounds and predict their properties. However, human understanding of chemical principles remains crucial for interpreting and applying these AI-generated insights effectively.
Connecting Chemistry to Daily Life
You might be surprised to learn how often you encounter chemistry related to formulas like HCOOCH CH₂ H₂O in your daily life. From the soap you use to wash your hands to the food you eat, chemical reactions and molecular structures are everywhere.
Cooking is actually applied chemistry. When you bake bread, chemical reactions transform simple ingredients into something completely different. Understanding molecular structures helps explain why certain ingredients work well together while others don’t.
Even cleaning your house involves chemistry. Detergents work by having molecules with both water-loving and oil-loving parts. This allows them to remove greasy stains that water alone cannot handle. It’s all about understanding how different molecular structures interact.
Building Your Chemical Vocabulary
As you become more comfortable with formulas like HCOOCH CH₂ H₂O, you’ll start recognizing common patterns and groups. This is like learning vocabulary in a new language. The more words you know, the easier it becomes to understand new sentences.
Start by learning about functional groups – common arrangements of atoms that appear in many different molecules. Once you can spot these groups, understanding complex formulas becomes much easier. It’s like recognizing familiar faces in a crowd.
Don’t be afraid to look things up when you encounter unfamiliar formulas. Every chemist, from students to Nobel Prize winners, regularly consults reference materials. The goal isn’t to memorize everything, but to understand the principles and know where to find information when you need it.
Remember, chemistry is all around us, and understanding formulas like HCOOCH CH₂ H₂O opens up a fascinating world of molecular interactions and possibilities. Keep exploring, stay curious, and don’t be afraid to ask questions!